Question Video: Calculating the Moles of a Gas in a Given Volume by Determining the Molar Gas Volume | Nagwa

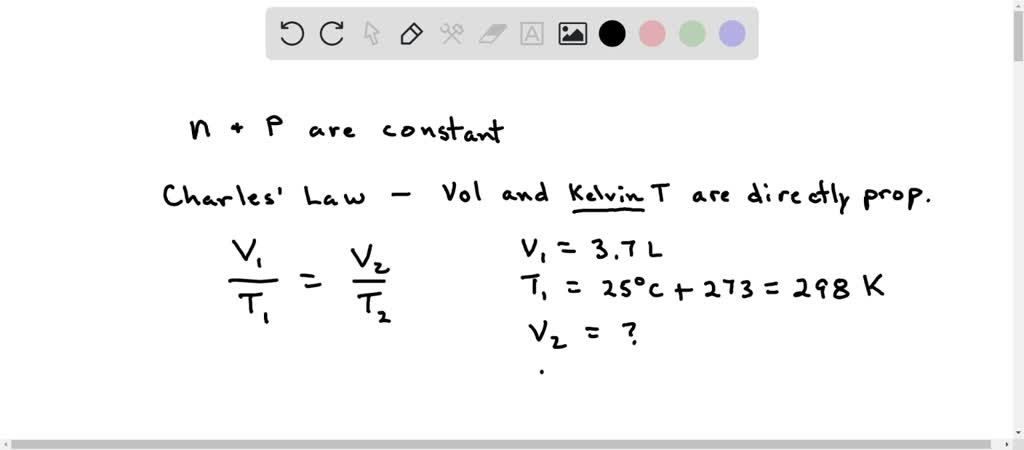
View question - A Helium filled weather balloon, when released, has a volume of 10.0 L at 27°C and a pressure of 663 mmHg. What Volume, in liters, will the balloon occupy at

A weather balloon has a volume of 175 L when filled with hydrogen at a pressure of 1.000 atm. Calculate the volume of the balloon when it rises to a height of

A balloon filled with helium gas at 1.00 atm occupies 11.1 L. What volume would the balloon occupy in the upper atmosphere, at a pressure of 0.25 atm and a constant temperature?
![Calculate from the Van der Waals equation, the temperature at which 192g of SO2 would occupy a volume of 6 dm^3 at 15 atm pressure [a = 5.68 atm L^2 mol^-2, b = 0.06 L mol^-1] Calculate from the Van der Waals equation, the temperature at which 192g of SO2 would occupy a volume of 6 dm^3 at 15 atm pressure [a = 5.68 atm L^2 mol^-2, b = 0.06 L mol^-1]](https://dwes9vv9u0550.cloudfront.net/images/2086003/05177f32-83c6-4e02-85a6-3b11431b23aa.jpg)
Calculate from the Van der Waals equation, the temperature at which 192g of SO2 would occupy a volume of 6 dm^3 at 15 atm pressure [a = 5.68 atm L^2 mol^-2, b = 0.06 L mol^-1]
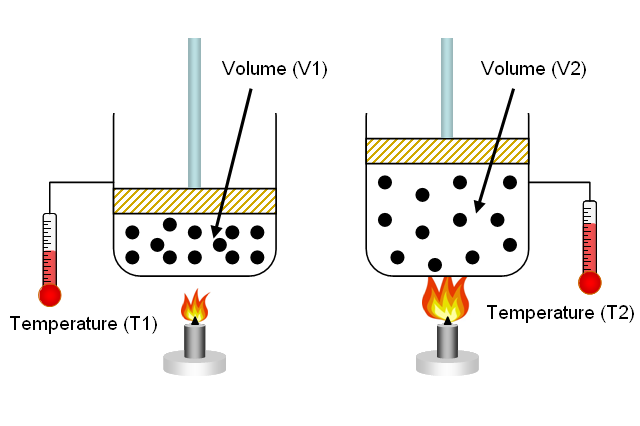
A balloon has a volume of 253.2 L at 356 K. The volume of the balloon is decreased to 165.4 L. What is the new temperature? | Socratic

SOLVED: A balloon has a volume of 1.80 liters at 24.0*C. The balloon is heated to 48.0*C. Calculate the new volume of the balloon a. 1.95 L b. 1.80 L c. 1.67

A helium-filled balloon at sea level has a volume of 2.10 L at atm and 36 C. If it is released and rises to an elevation at which the pressure is. -

A balloon has a volume of 3.00 liters at 24.0°c. the balloon is heated to 48.0°c. calculate the new volume - Brainly.com