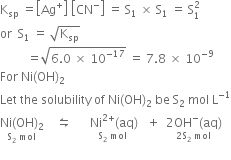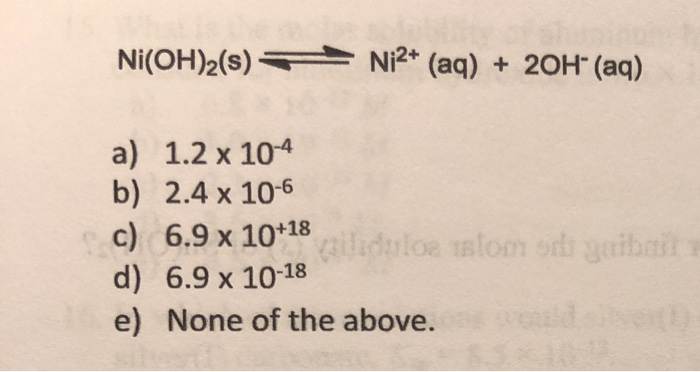Plots of log S of Ni(OH) 2 , Fe(OH) 2 , and Co(OH) 2 as a function of... | Download Scientific Diagram

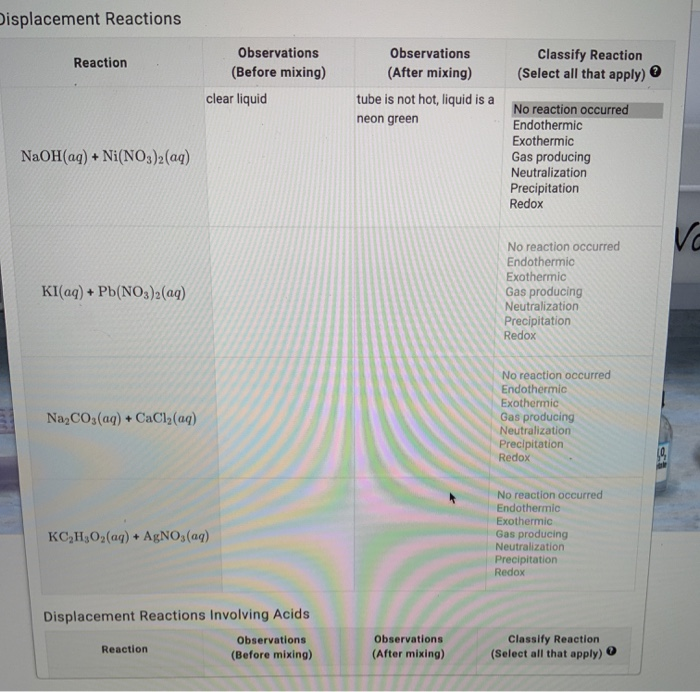
Major soluble Ni-species of 0.1 mM NiSO 4 added to pure water at 25 ◦... | Download Scientific Diagram

Solubility of NiO (cr) at infinite dilution versus pH° m at temperature... | Download Scientific Diagram

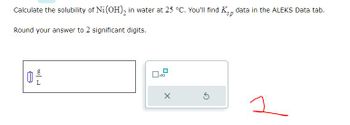
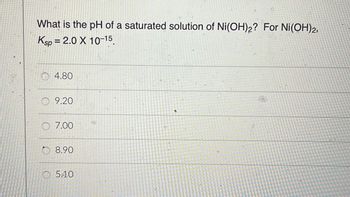
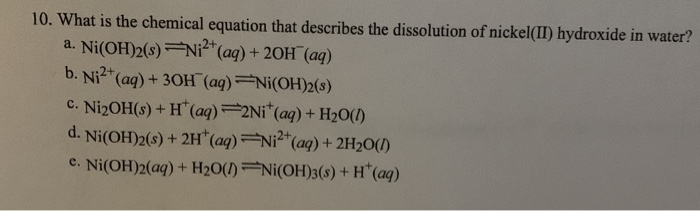
The solubility product of `Ni(OH)_(2)` is `2.0xx10^(-15)`. The molar solubility of `Ni(OH)_(2)` ... - YouTube
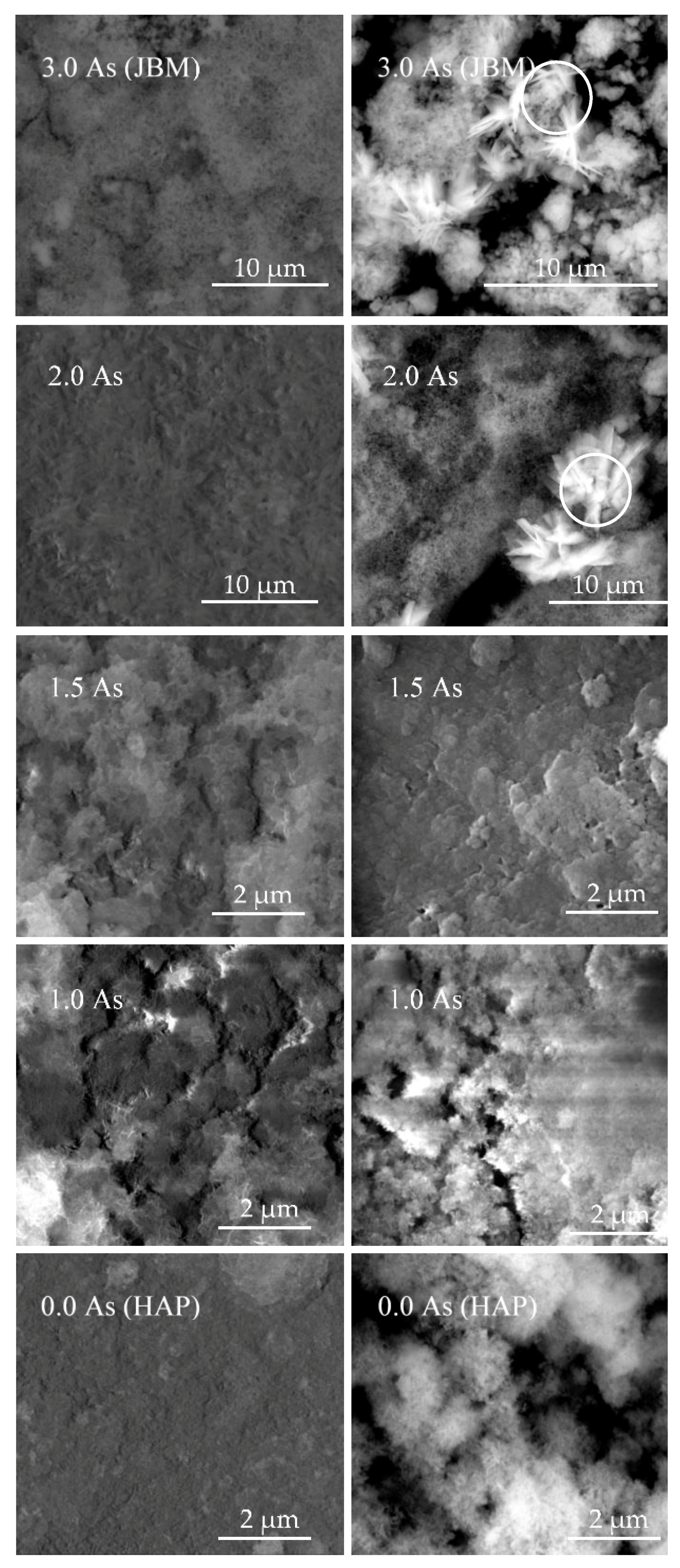
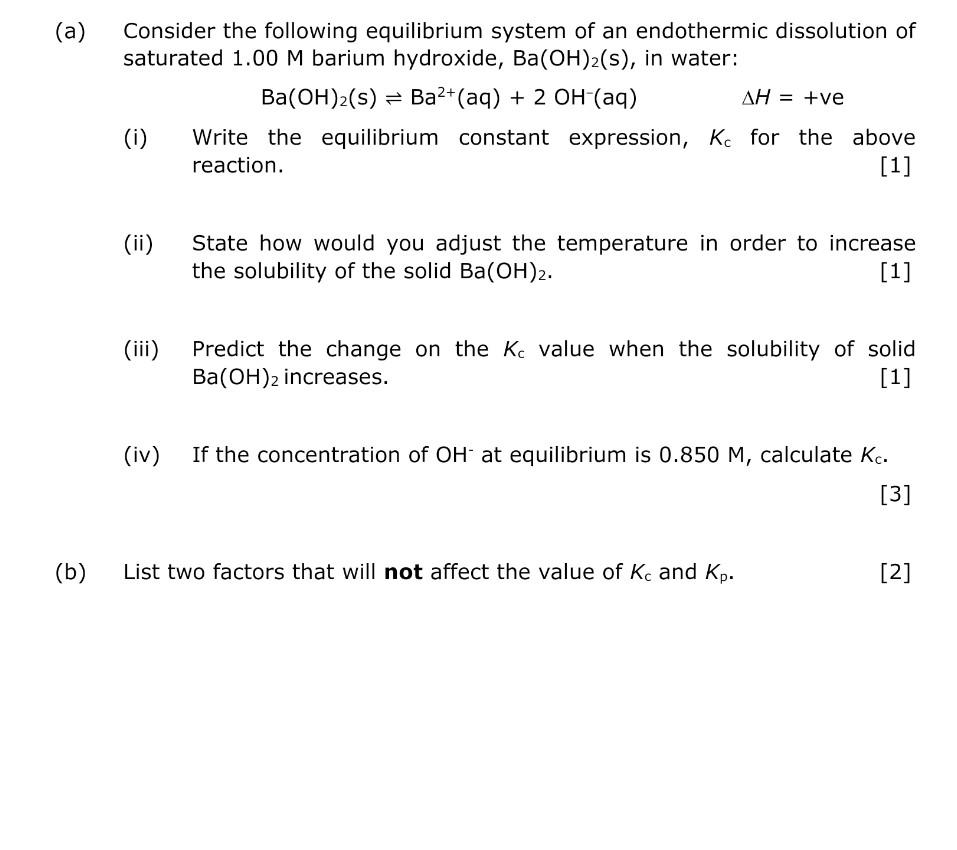
Minerals | Free Full-Text | Transition from Endothermic to Exothermic Dissolution of Hydroxyapatite Ca5(PO4)3OH–Johnbaumite Ca5(AsO4)3OH Solid Solution Series at Temperatures Ranging from 5 to 65 °C

The solubility product of `Ni(OH)_(2)` is `2.0xx10^(-15)`. The molar solubility of `Ni(OH)_(2)` ... - YouTube