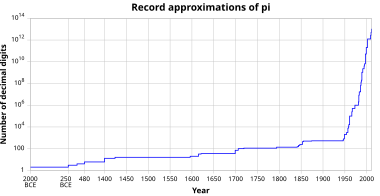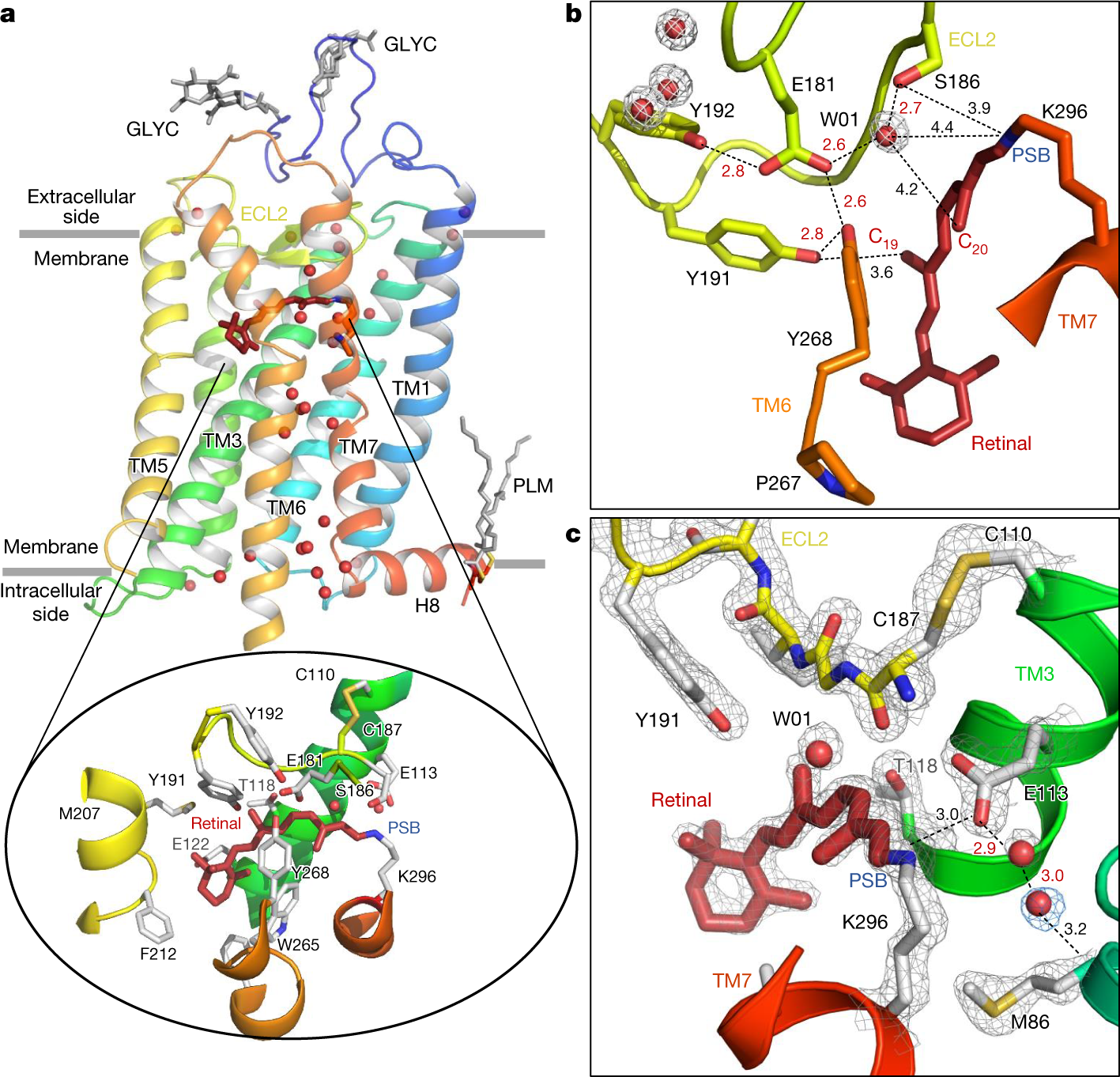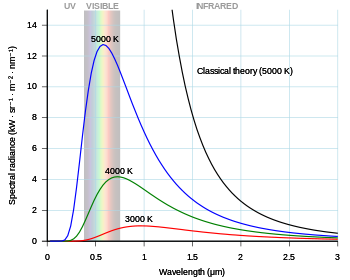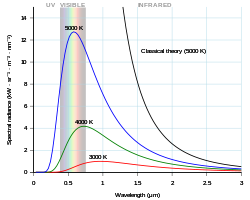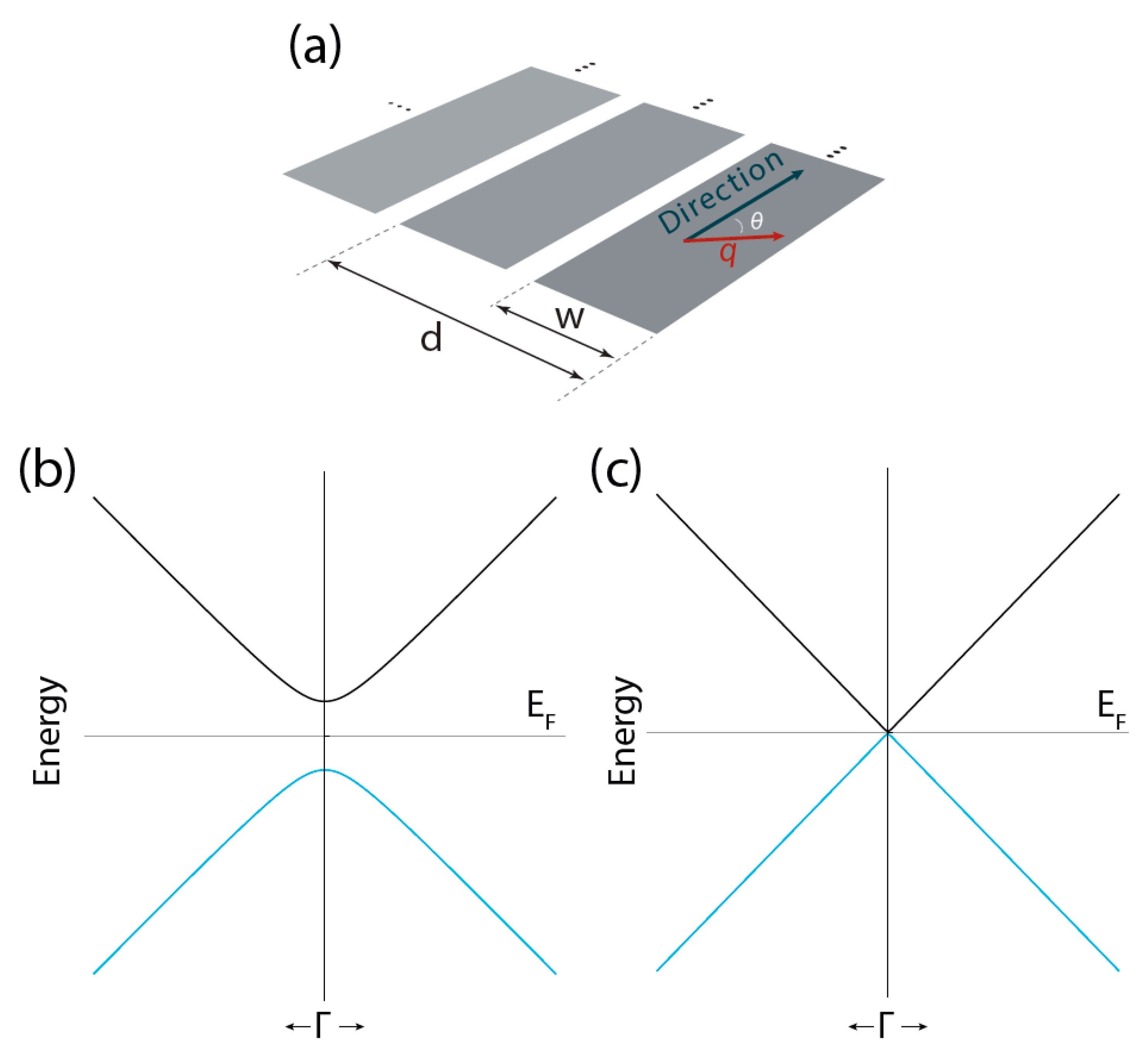
Chemosensors | Free Full-Text | Proving Surface Plasmons in Graphene Nanoribbons Organized as 2D Periodic Arrays and Potential Applications in Biosensors

Antioxidants | Free Full-Text | The Influence of 5′,8-Cyclo-2′-Deoxyguanosine on ds-DNA Charge Transfer Depends on Its Diastereomeric Form: A Theoretical Study
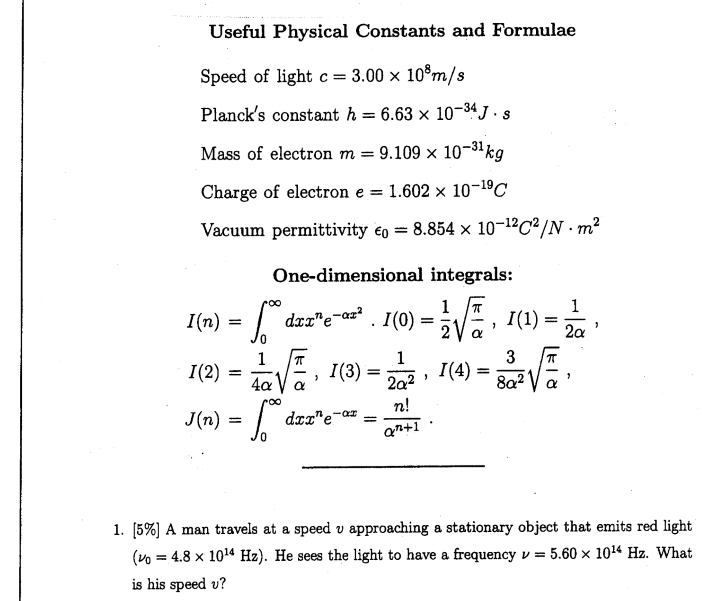
The value of Planck's constant is `6.63 xx 10^(-34)Js`. The speed of light is `3xx10^(17)nm s^ - YouTube

Planck's constant (h), speed of light in vacuum (c) and Newton's gravitational constant (G) are three fundamental constants. Which of the following combinations of these has the dimension of length?
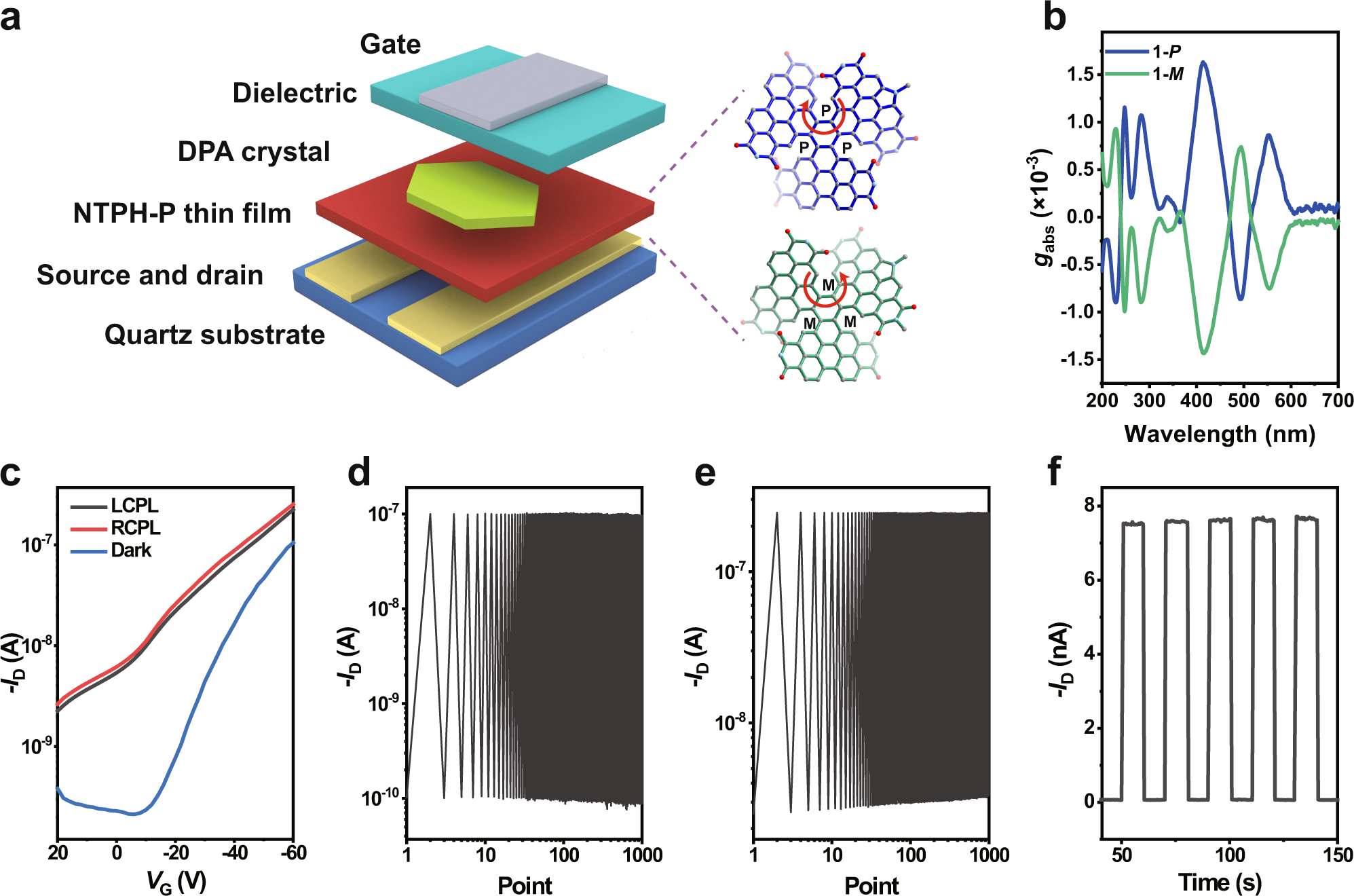
Organic donor-acceptor heterojunctions for high performance circularly polarized light detection | Nature Communications
![Calculate the energy of the light having wavelength 45 nm : [Planck's constant h = 6.63 × 10^-34 Js ; speed of light c = 3 × 10^8 ms^-1 ] Calculate the energy of the light having wavelength 45 nm : [Planck's constant h = 6.63 × 10^-34 Js ; speed of light c = 3 × 10^8 ms^-1 ]](https://dwes9vv9u0550.cloudfront.net/images/8714373/032957da-34c2-47ec-a991-027613566c64.jpg)
Calculate the energy of the light having wavelength 45 nm : [Planck's constant h = 6.63 × 10^-34 Js ; speed of light c = 3 × 10^8 ms^-1 ]

The value of Planck's constant (h) is 6.63 × 10^-34 Js . The velocity of light is 3.0 × 10^8 ms^-1 . Which value is closest to the wavelength (in meters) of

Fine-Structure Constant Connects Electronic Polarizability and Geometric van-der-Waals Radius of Atoms | The Journal of Physical Chemistry Letters