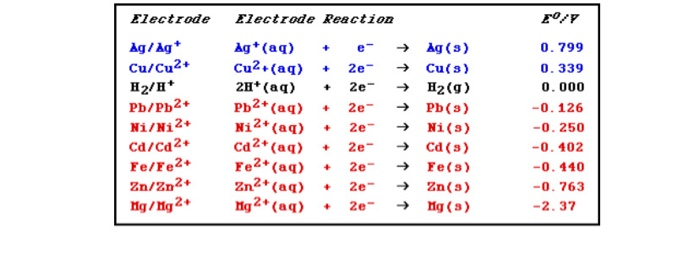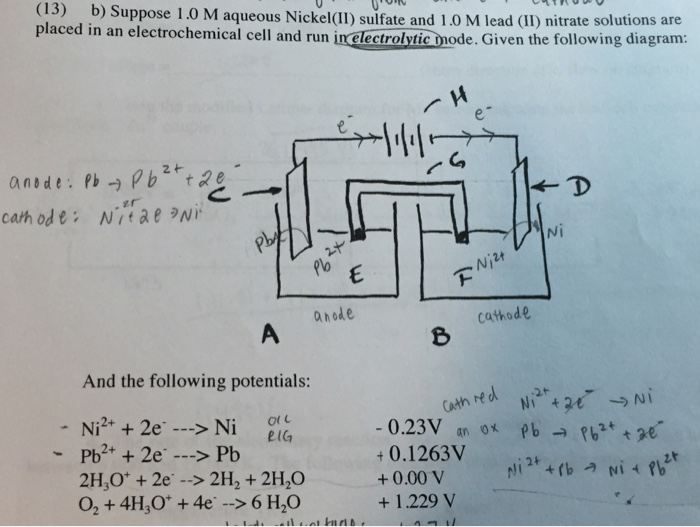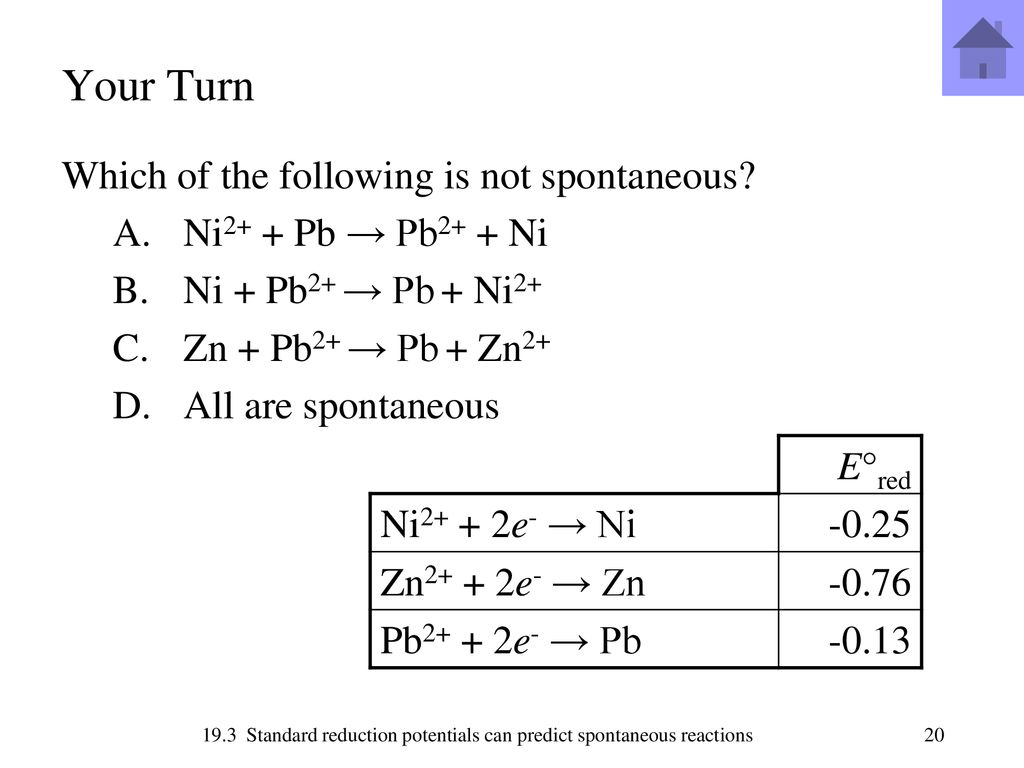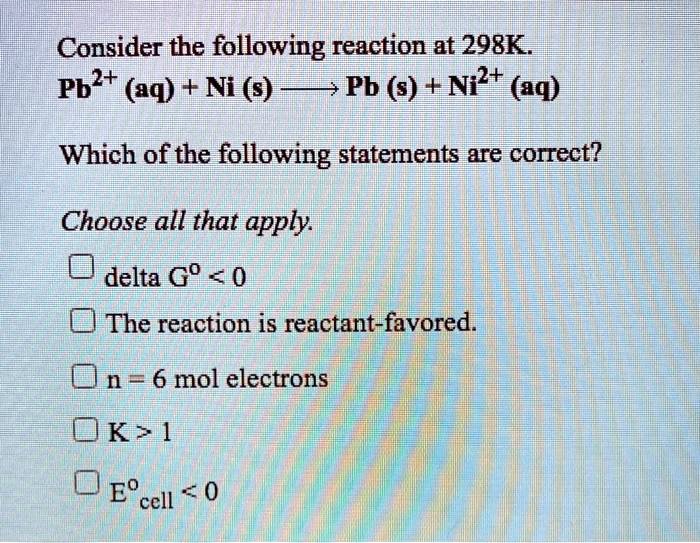
SOLVED: Consider the following reaction at 298K. Pb2+ (aq) + Ni (s) Pb (s) + Ni2+ (aq) Which of the following statements are correct? Choose all that apply: Odelta G' < 0
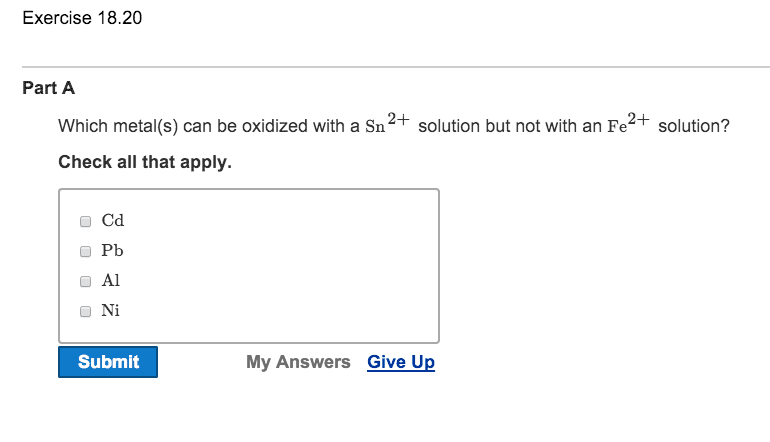
OneClass: Which metal(s) can be oxidized with a Sn^2+ solution but not with an Fe^2+ solution? Check ...
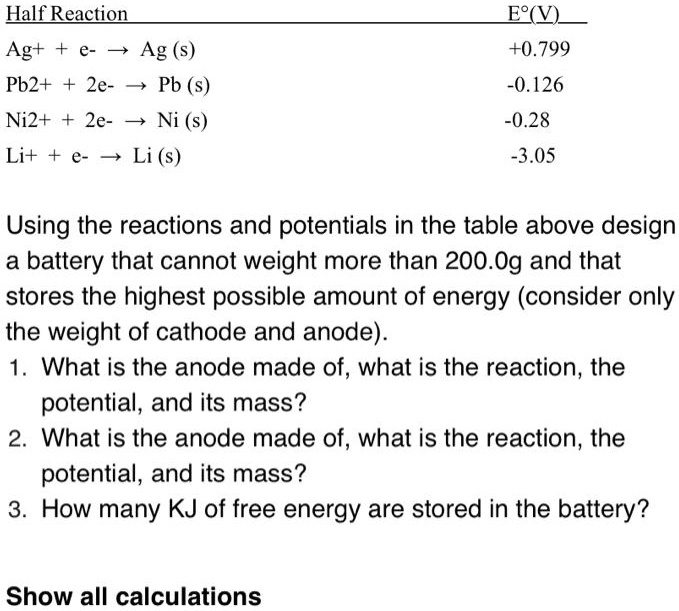
SOLVED: Half Reaction Agt 3 Ag (s) Pb2+ 2e- 4 Pb (s) Ni2+ + 2e- 5 Ni (s) Li+ P Li (s) F?(V) +0.799 -0.126 -0.28 -3.05 Using the reactions and potentials
The effective removal of Ni2+, Cd2+, and Pb2+ from aqueous solution by adenine-based nano-adsorbent - RSC Advances (RSC Publishing)
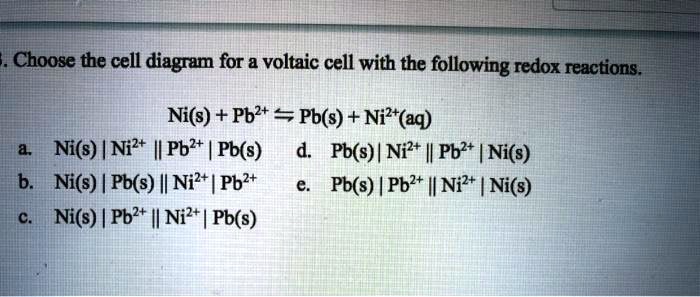
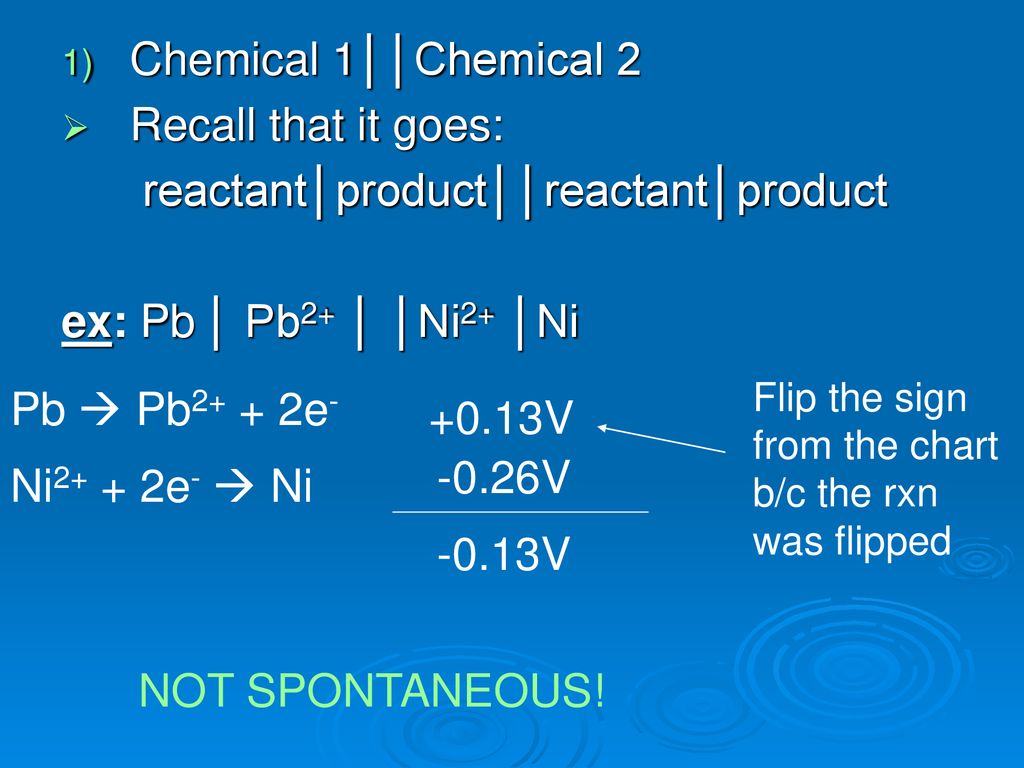
SOLVED: Choose the cell diagram for voltaic cell with the following redox reactions. Ni(s) + Pb2+ == Pb(s) + Ni?*(aq) Ni(s) | Nil+ I Pbl+ Pb(s) Pb(s) | Ni2+ |l Pb2+

The adsorbent Brassica stem comparison on adsorption of Ni (II), Cr... | Download Scientific Diagram

The Use of Adansonia digitata L. Seed Cake Residue as a Biosorbent in the Bioremediation of Lead (Pb2+) from Aqueous Solution | Semantic Scholar

Preparation of novel terephthalic acid modified Fe/Ni metal organic nanosheet with high adsorption performance for trace Pb2+ - ScienceDirect

Sketch a voltaic cell for this redox reaction: Ni^{2+} (aq) + Mg (s) to Ni (s) + Mg^{2 +}(aq) a. Label the anode and cathode. b. Write the half reactions. c. Indicate