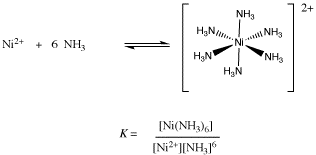Crystals | Free Full-Text | Ammonia Uptake and Release in the MnX2–NH3 (X = Cl, Br) Systems and Structure of the Mn(NH3)nX2 (n = 6, 2) Ammines
Draw the structures of [Co(NH3)6]^3+, [Ni(CN)4]^2- and [Ni(CO)4]. - Sarthaks eConnect | Largest Online Education Community
![Cr(NH3)6)3+ is paramagnetic while [Ni(CN)4]2 – is diamagnetic. Explain why? from Chemistry Coordination Compounds Class 12 Assam Board Cr(NH3)6)3+ is paramagnetic while [Ni(CN)4]2 – is diamagnetic. Explain why? from Chemistry Coordination Compounds Class 12 Assam Board](https://www.zigya.com/application/zrc/images/qvar/CHEN12070288.png)
Cr(NH3)6)3+ is paramagnetic while [Ni(CN)4]2 – is diamagnetic. Explain why? from Chemistry Coordination Compounds Class 12 Assam Board
![Explain the type hybridisation, magnetic property and geometry for [ Ni (CN)4 ]^2 - and [ Ni (NH3)4 ]^2 + using VB theory. Explain the type hybridisation, magnetic property and geometry for [ Ni (CN)4 ]^2 - and [ Ni (NH3)4 ]^2 + using VB theory.](https://haygot.s3.amazonaws.com/questions/633645_607318_ans_06ef044c85004ad2aed957fc1fd1f24d.png)
Explain the type hybridisation, magnetic property and geometry for [ Ni (CN)4 ]^2 - and [ Ni (NH3)4 ]^2 + using VB theory.
Draw the structures of [Co(NH3)6]^3+, [Ni(CN)4]^2- and [Ni(CO)4]. - Sarthaks eConnect | Largest Online Education Community
![Under the valence bond approach explain the shape and magnetic behaviour of [Ni(NH3)6]2+.[Given At. No. of Ni = 28] - Wired Faculty Under the valence bond approach explain the shape and magnetic behaviour of [Ni(NH3)6]2+.[Given At. No. of Ni = 28] - Wired Faculty](https://www.wiredfaculty.com/application/zrc/images/qvar/CHEN12070315.png)
Under the valence bond approach explain the shape and magnetic behaviour of [Ni(NH3)6]2+.[Given At. No. of Ni = 28] - Wired Faculty
![Exceptional case, Hybridisation ,shape of an outer orbital complex [ Ni(NH3) 6]2+, Octahedral complex - YouTube Exceptional case, Hybridisation ,shape of an outer orbital complex [ Ni(NH3) 6]2+, Octahedral complex - YouTube](https://i.ytimg.com/vi/R5RDFu1oYUU/maxresdefault.jpg)
![How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-61bcbbeed69e0a294c52675e216d428f.webp)

![Answered: [Ni (NH3) 6] C12 Explain the… | bartleby Answered: [Ni (NH3) 6] C12 Explain the… | bartleby](https://content.bartleby.com/qna-images/question/33dc3186-d7b9-4388-8560-215f799e8fe0/013f6e76-4df4-4063-bb5c-5eb17a580f72/z92qakc_thumbnail.png)
![Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni(NH3)6]^( Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni(NH3)6]^(](https://d10lpgp6xz60nq.cloudfront.net/physics_images/VIK_CHE_QB_C07_E04_030_S01.png)
![Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni(NH3)6]^( Why [Co(NH3)6]^(3+) is an inner orbital complex where is [Ni(NH3)6]^(](https://d10lpgp6xz60nq.cloudfront.net/physics_images/VIK_CHE_QB_C07_E04_030_S02.png)
![How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora How is [Ni(NH3) 6] 2+ paramagnetic while [Ni(CN) 6] 4- diamagnetic? - Quora](https://qph.cf2.quoracdn.net/main-qimg-f7fd36508df0305723aa4956637d3097.webp)
![PDF) Synthesis, structure and thermal decomposition of [Ni(NH3) 6][VO(O2)2( NH3)]2 PDF) Synthesis, structure and thermal decomposition of [Ni(NH3) 6][VO(O2)2( NH3)]2](https://i1.rgstatic.net/publication/256461217_Synthesis_structure_and_thermal_decomposition_of_NiNH3_6VOO22NH32/links/598c15b1458515c333a5e1ff/largepreview.png)

![Answered: [Ni (NH3) 6] Magnetic moment of 2+… | bartleby Answered: [Ni (NH3) 6] Magnetic moment of 2+… | bartleby](https://content.bartleby.com/qna-images/answer/c73807b6-45ed-4420-a3b1-cee7bec70749/203ec893-0265-480e-b058-95e1378e904d/3cda3a30-aca8-11eb-99b8-c16e6153b0d2_IMG_20210504_124206_523~2.jpg)
![Characteristic bands in infrared spectrum of [Ni(NH3) 6 ][VO(O 2 ) 2... | Download Table Characteristic bands in infrared spectrum of [Ni(NH3) 6 ][VO(O 2 ) 2... | Download Table](https://www.researchgate.net/publication/256461217/figure/tbl1/AS:667594596024340@1536178363556/Characteristic-bands-in-infrared-spectrum-of-NiNH3-6-VOO-2-2-NH-3-2.png)