
Concentration change of Fe, Cr and Ni in NaOH solution of pH 13 after... | Download Scientific Diagram

Destruction of PCBs mixture, content of biphenyls in reaction mixture... | Download Scientific Diagram

Destruction of PCBs mixture, content of biphenyls in reaction mixture... | Download Scientific Diagram

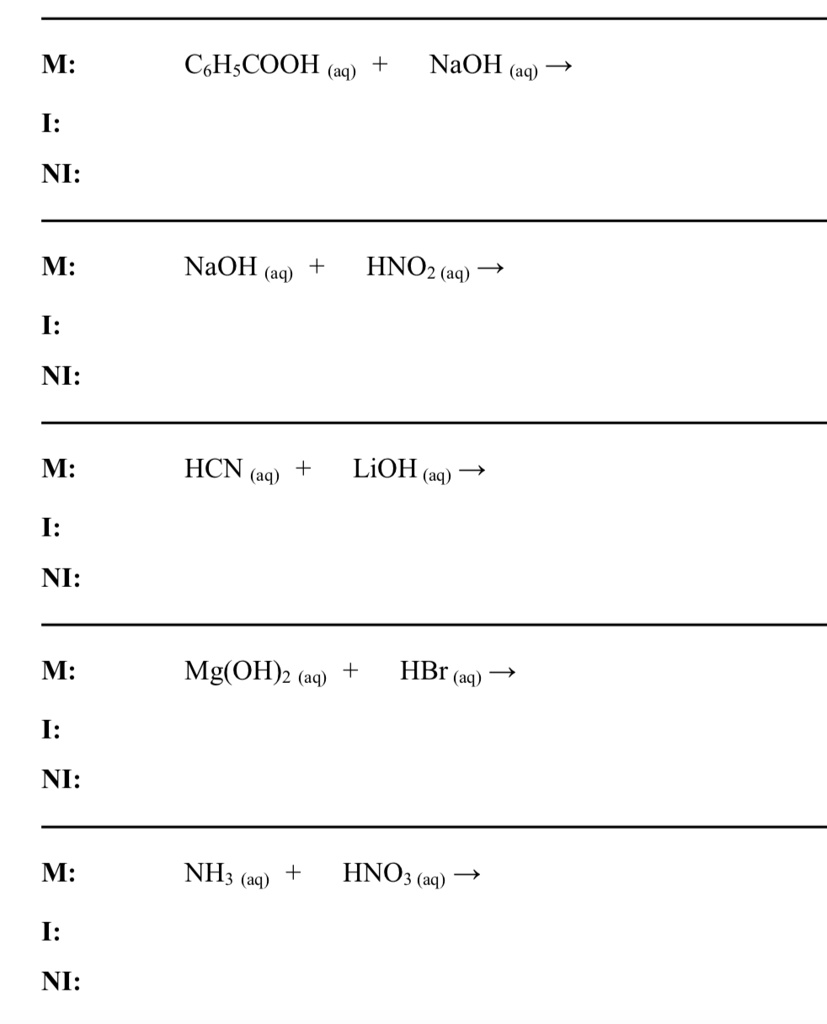
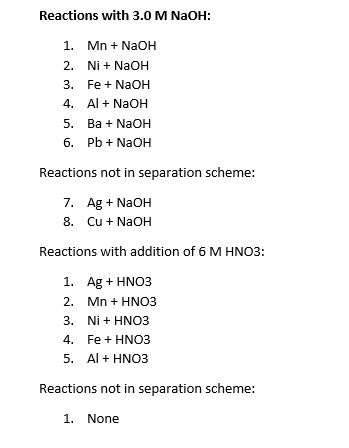
Chapter 4: Aqueous Reactions Solution: Solvent: substance present in the larger amount Solute: substance(s) dissolved in solvent, generally present in. - ppt download
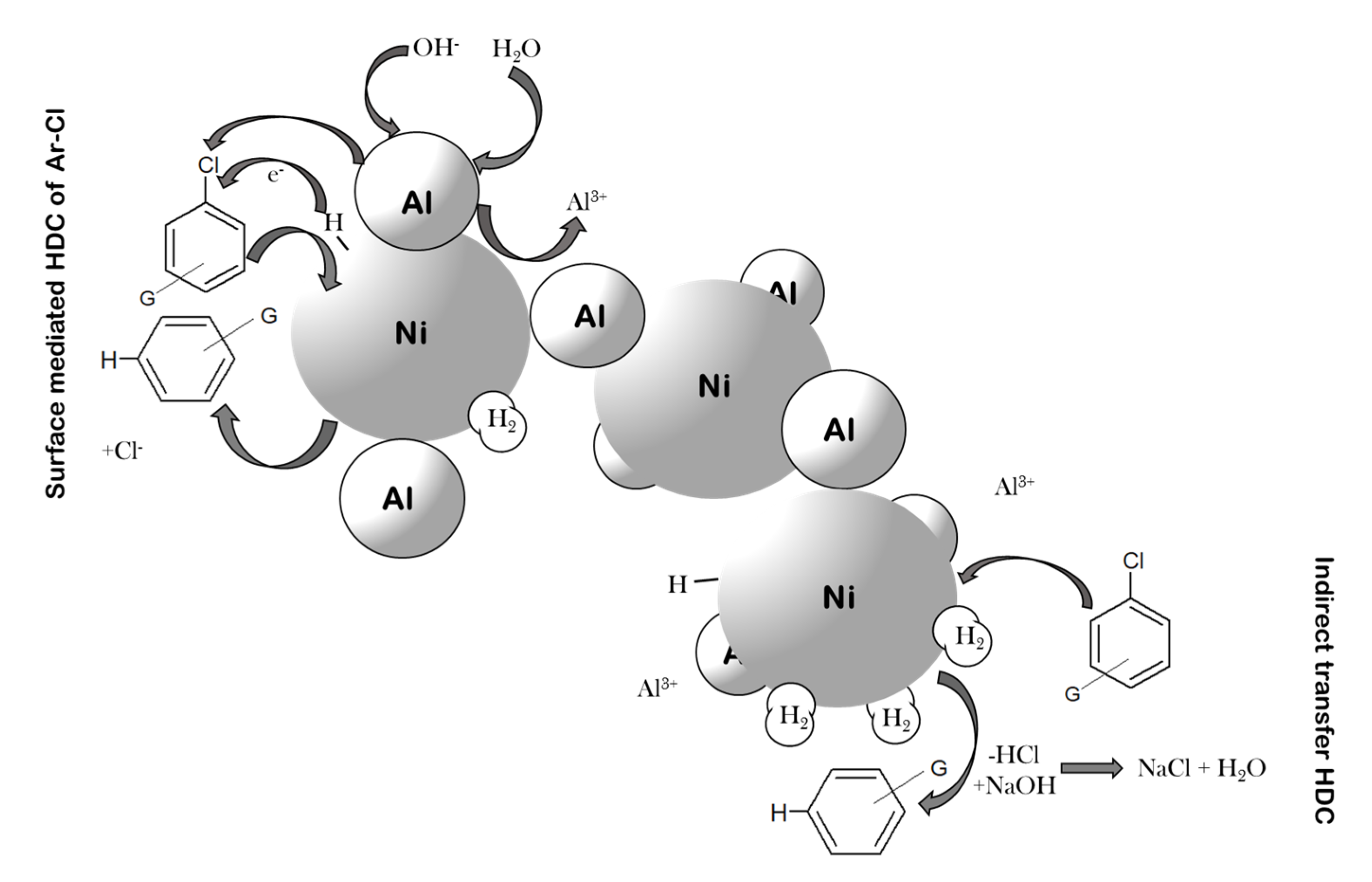
Catalysts | Free Full-Text | Hydrodechlorination of Different Chloroaromatic Compounds at Room Temperature and Ambient Pressure—Differences in Reactivity of Cu- and Ni-Based Al Alloys in an Alkaline Aqueous Solution

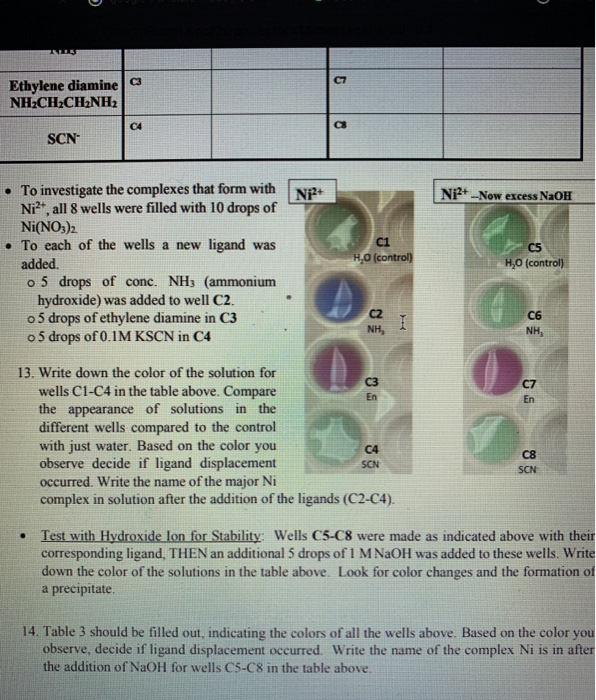
Nickel transition metal Chemistry nickel(II) Ni2+ complex ions ligand substitution redox chemical reactions principal oxidation states +2 +3 GCE AS A2 IB A level inorganic chemistry revision notes




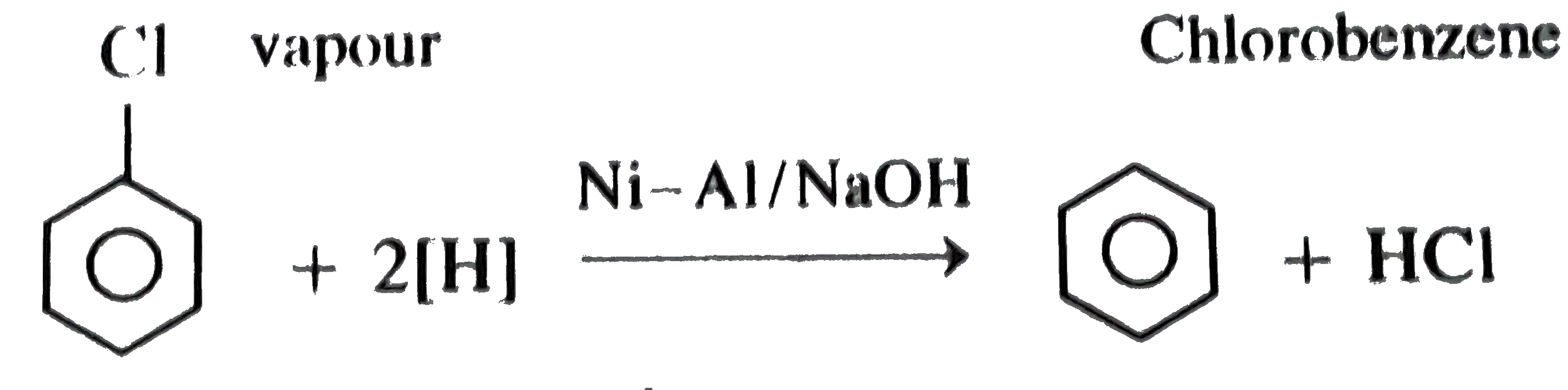
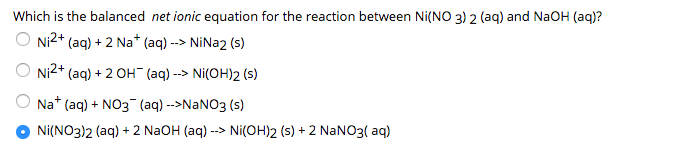





![C6H5Cl [ ]Ni - Al/NaOH (A) . In this reaction, (A) is: C6H5Cl [ ]Ni - Al/NaOH (A) . In this reaction, (A) is:](https://i.ytimg.com/vi/RQ0gxx5ezxk/maxresdefault.jpg)
