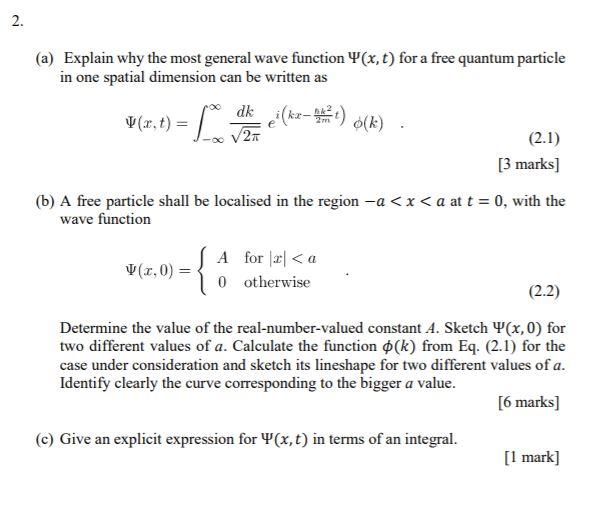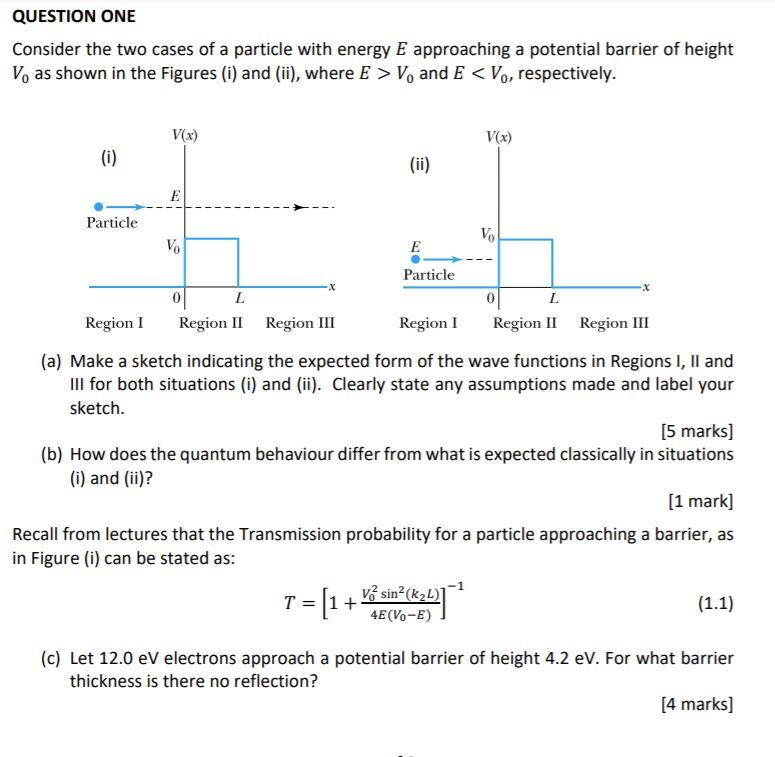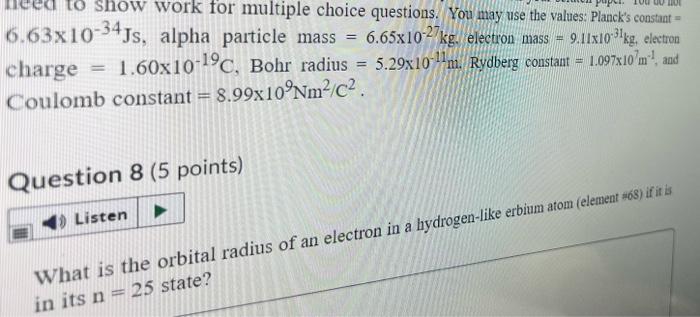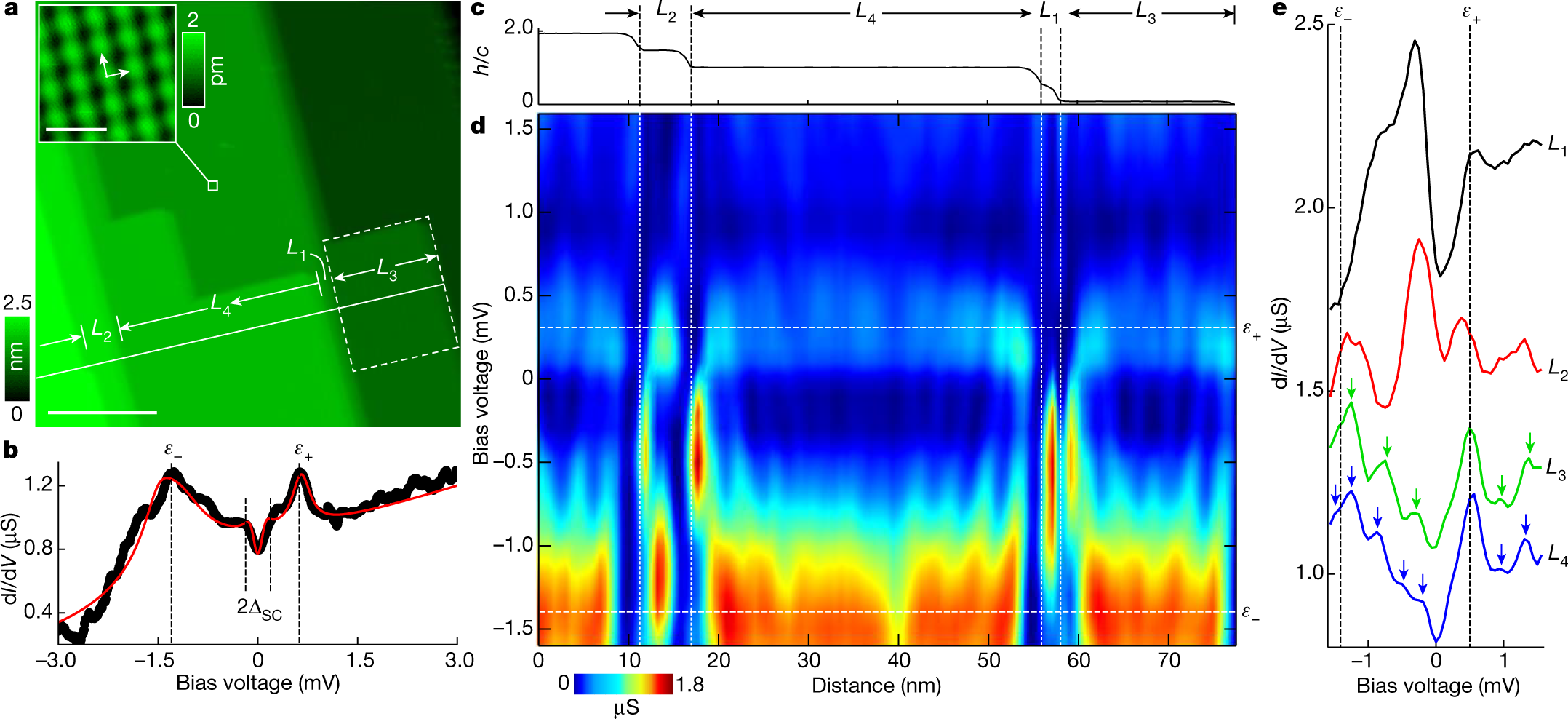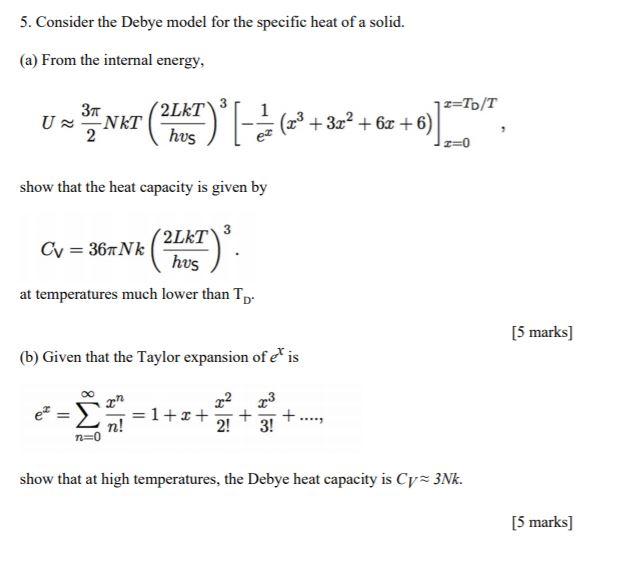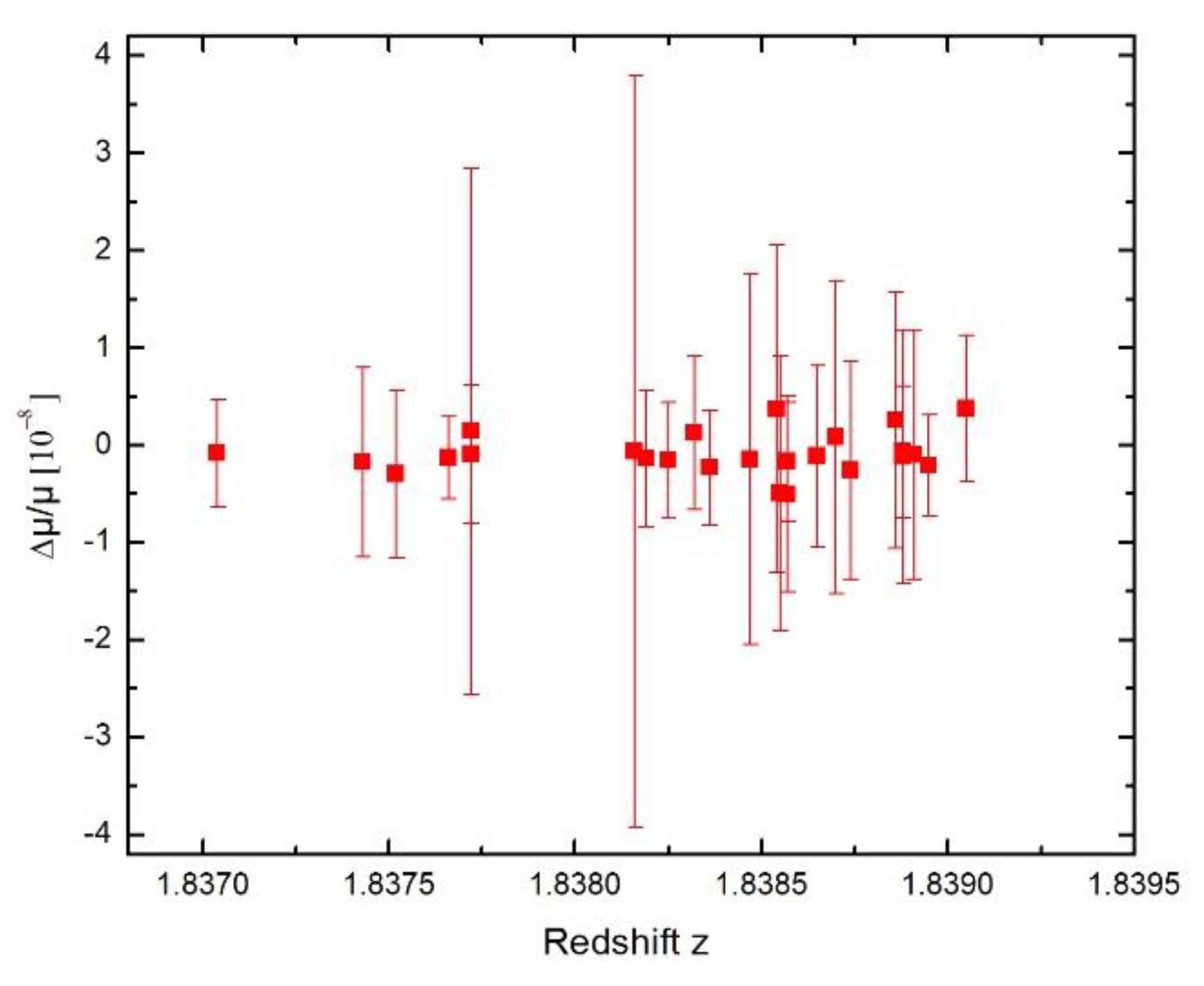
Symmetry | Free Full-Text | New Limit on Space-Time Variations in the Proton-to-Electron Mass Ratio from Analysis of Quasar J110325-264515 Spectra

Uncertainty in the position of an electron (mass = 9.1 × 10^-31kg) moving with a velocity 300ms^-1 accurate upon 0.001

Calculate the de - Broglie wavelength of an electron moving with one fifth of the speed of light. Neglect relativistic effects. ( h = 6.63 × 10^-34 J.s., c = 3 × 10^8 m/s , mass of electron = 9 × 10^-31 kg )

Physics of electron emission and injection in two‐dimensional materials: Theory and simulation - Ang - 2021 - InfoMat - Wiley Online Library
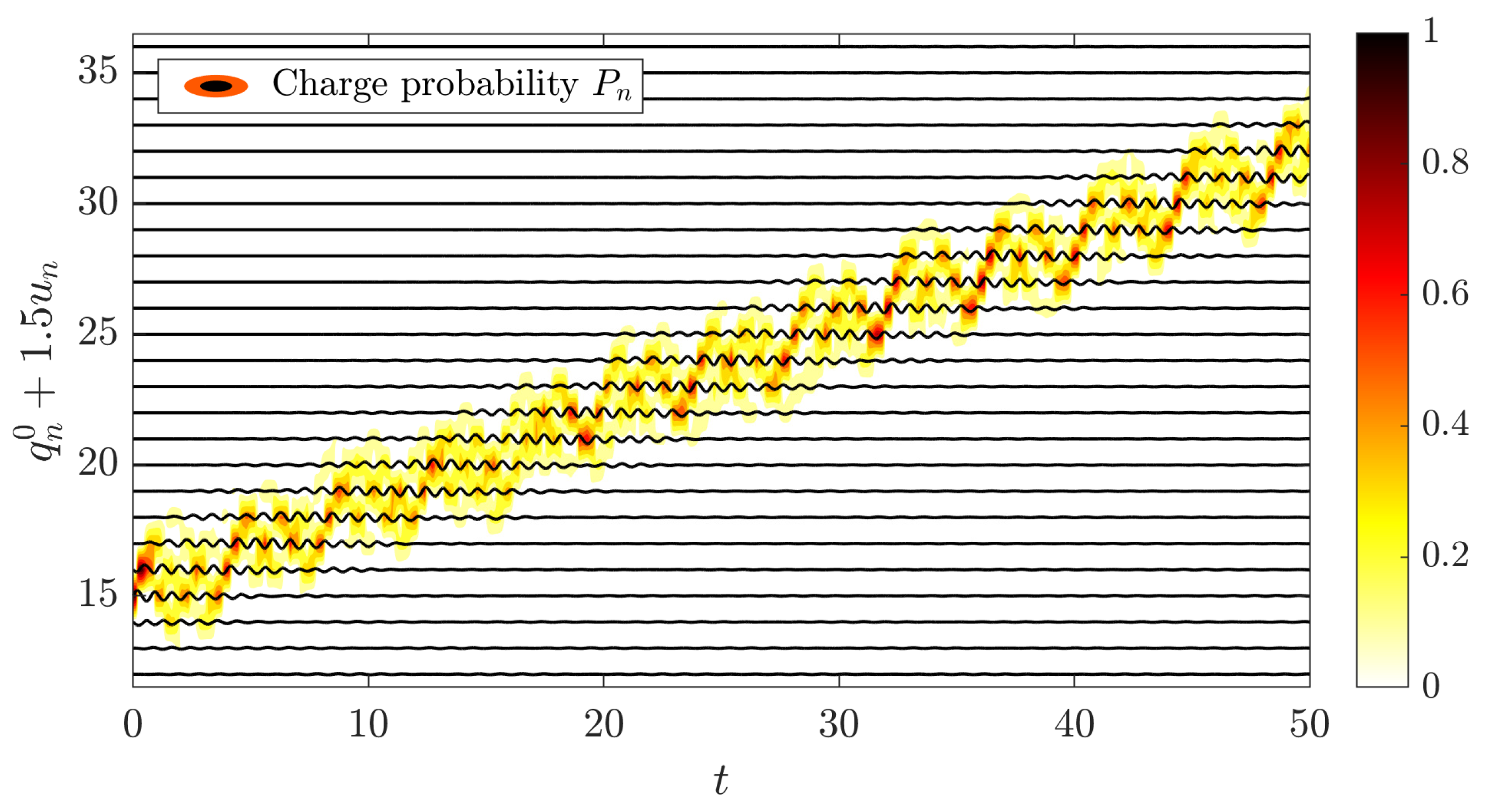
Mathematics | Free Full-Text | Splitting Methods for Semi-Classical Hamiltonian Dynamics of Charge Transfer in Nonlinear Lattices

Toward Detection of the Molecular Parity Violation in Chiral Ru(acac)3 and Os(acac)3 | The Journal of Physical Chemistry Letters
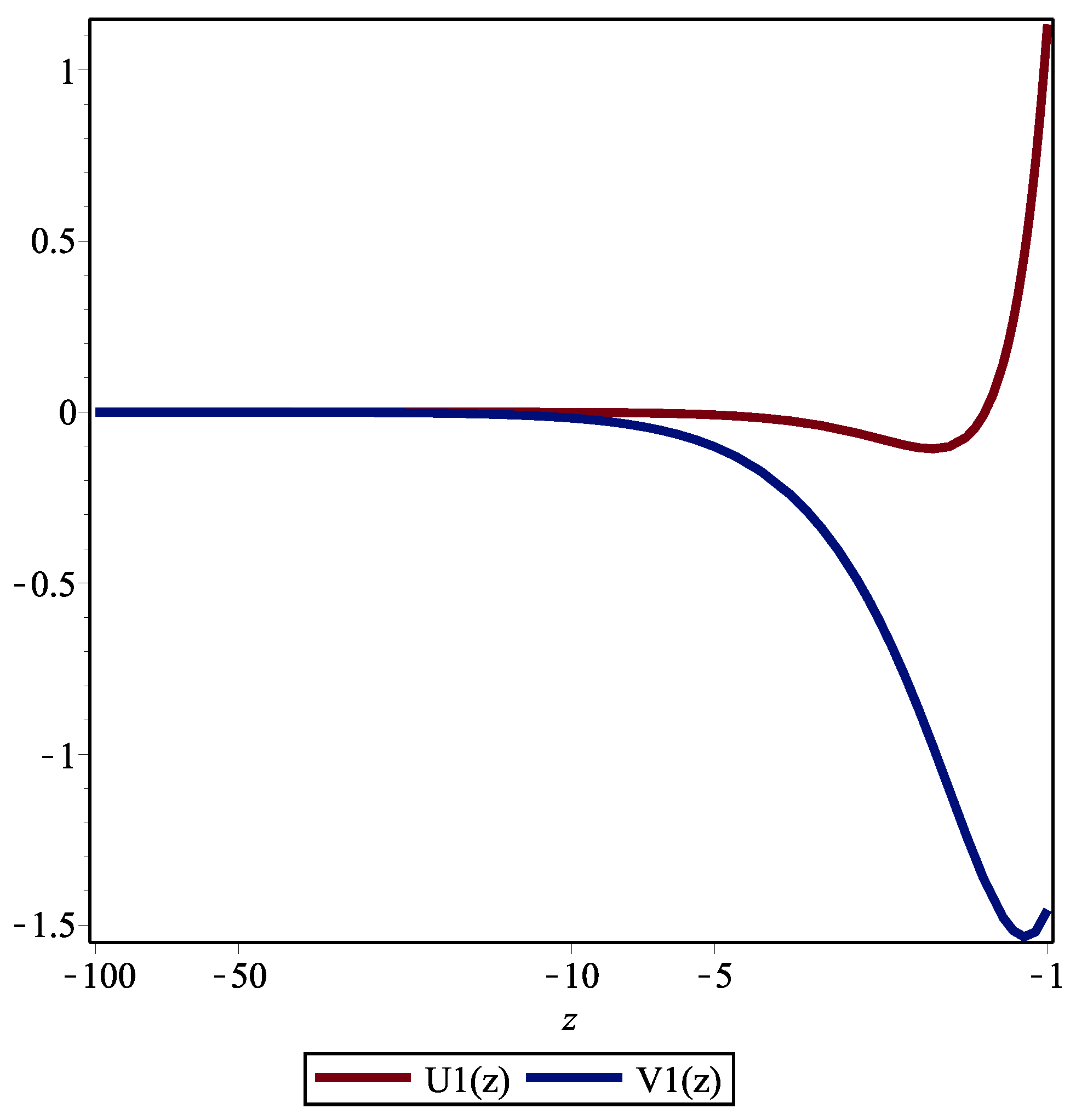
AppliedMath | Free Full-Text | Approximate Nonlocal Symmetries for a Perturbed Schrödinger Equation with a Weak Infinite Power-Law Memory
![Calculate the energy of the light having wavelength 45 nm : [Planck's constant h = 6.63 × 10^-34 Js ; speed of light c = 3 × 10^8 ms^-1 ] Calculate the energy of the light having wavelength 45 nm : [Planck's constant h = 6.63 × 10^-34 Js ; speed of light c = 3 × 10^8 ms^-1 ]](https://dwes9vv9u0550.cloudfront.net/images/8714373/032957da-34c2-47ec-a991-027613566c64.jpg)
Calculate the energy of the light having wavelength 45 nm : [Planck's constant h = 6.63 × 10^-34 Js ; speed of light c = 3 × 10^8 ms^-1 ]

The mass of electron is 9.11 × 10^-31 kg.Planck's constant is 6.626 × 10^-34 Js then the uncertainty involved in the measurement of velocity within a distance of 0.1 A is :

Given: The mass of electron is `9.11 � 10^(�31)`Kg Planck constant is `6.626 �10^(�34)` is:- - YouTube