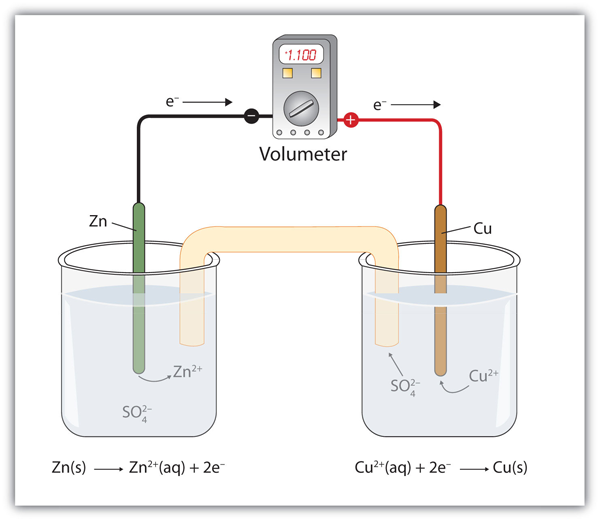
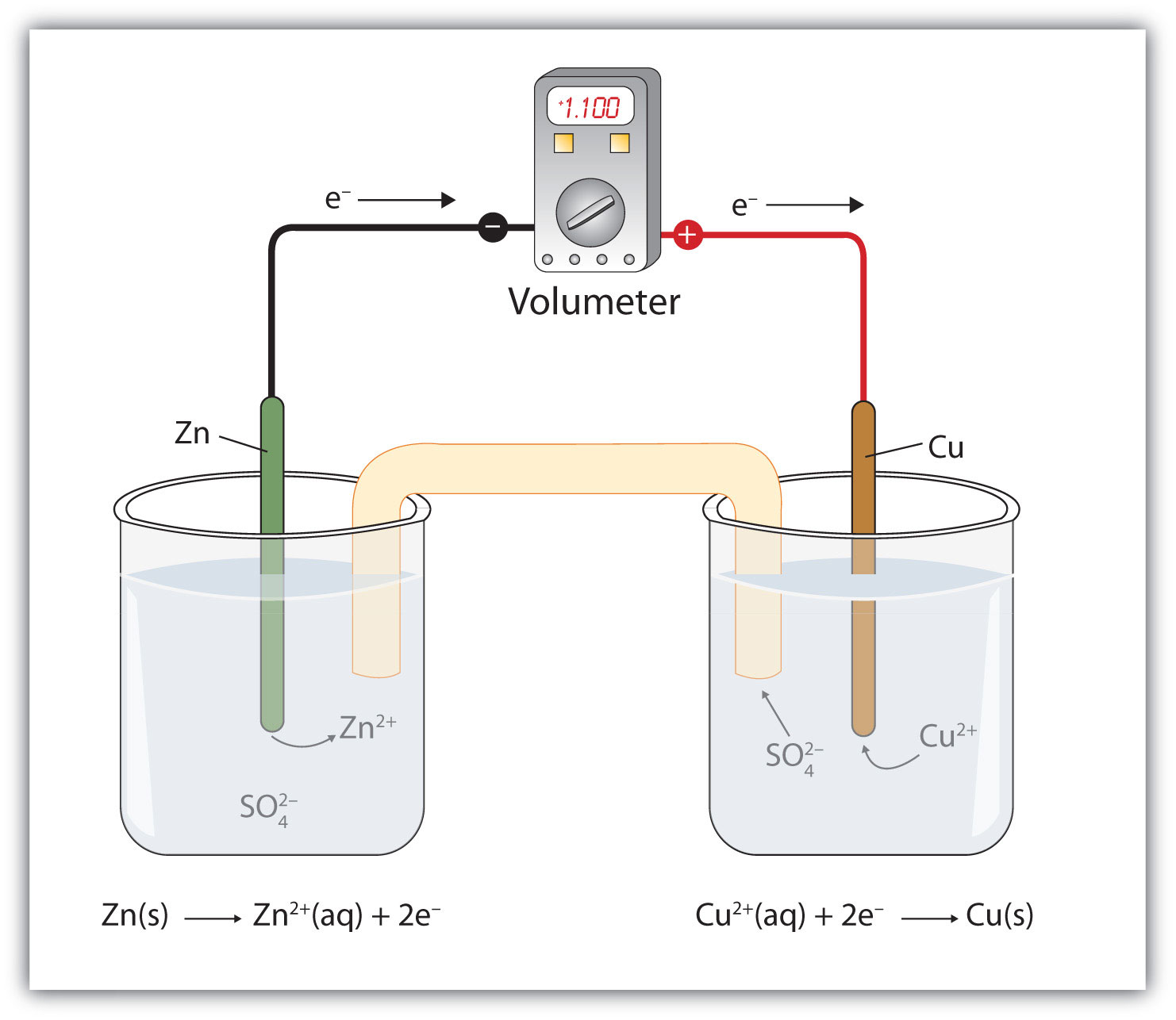
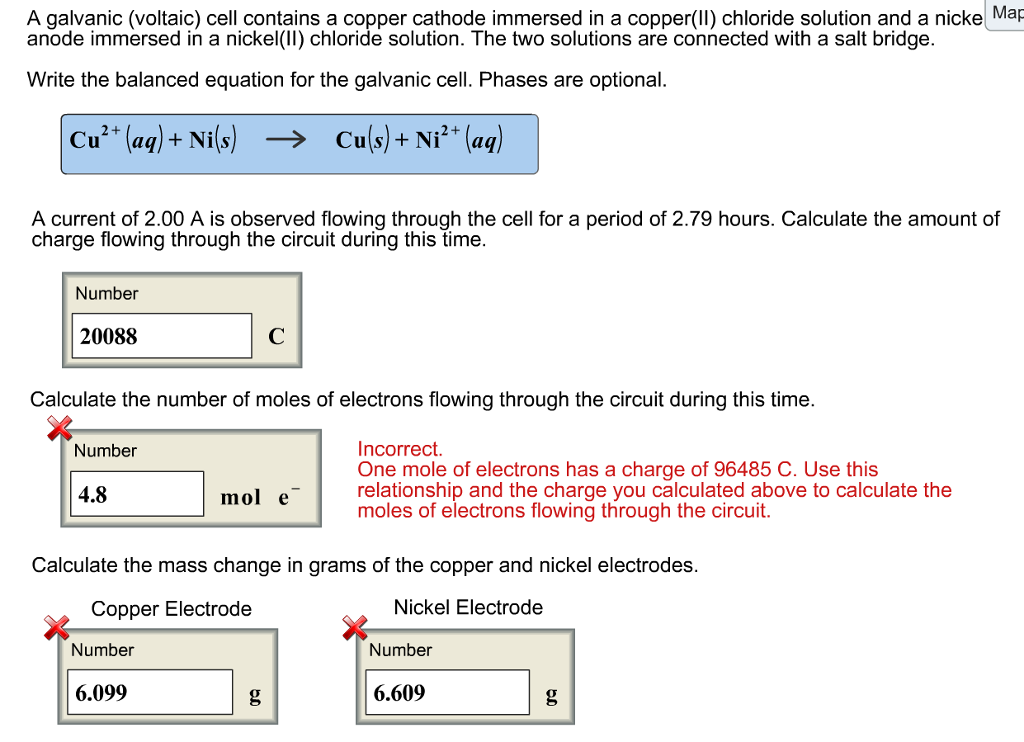
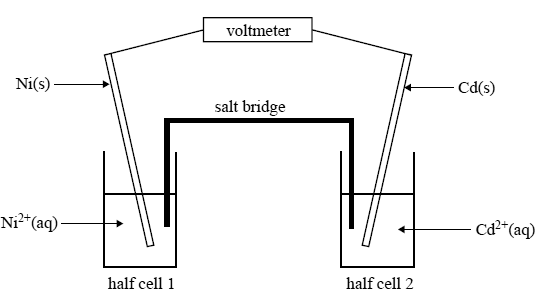
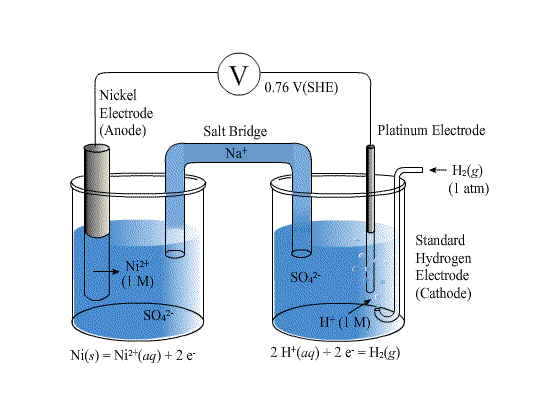
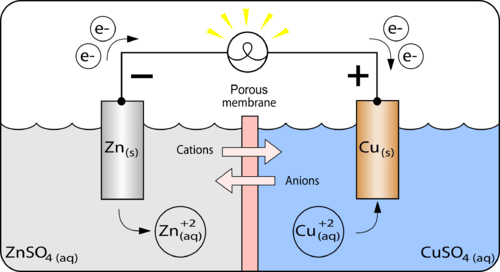
A galvanic cell is made up of a copper electrode in a 1.0 M copper (II) sulfate solution, a silver electrode in a 1.0 M silver nitrate solution, and a salt bridge

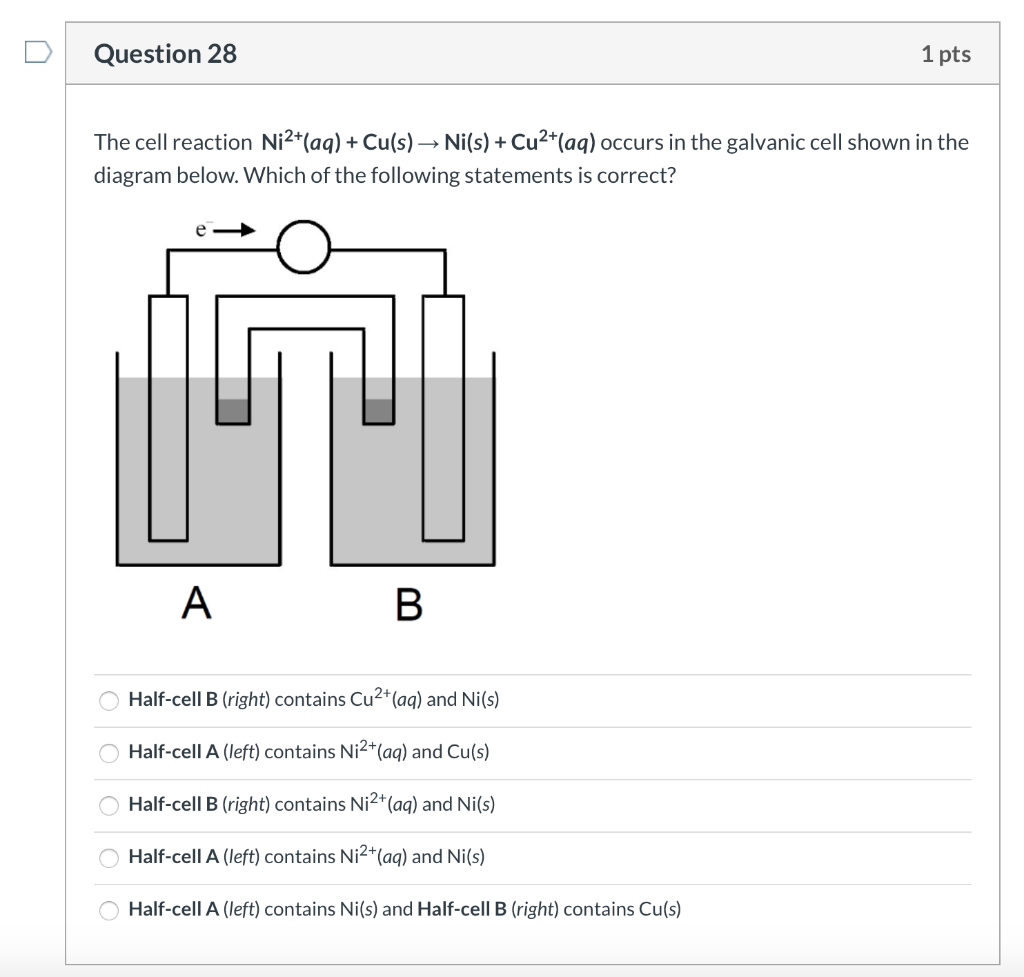
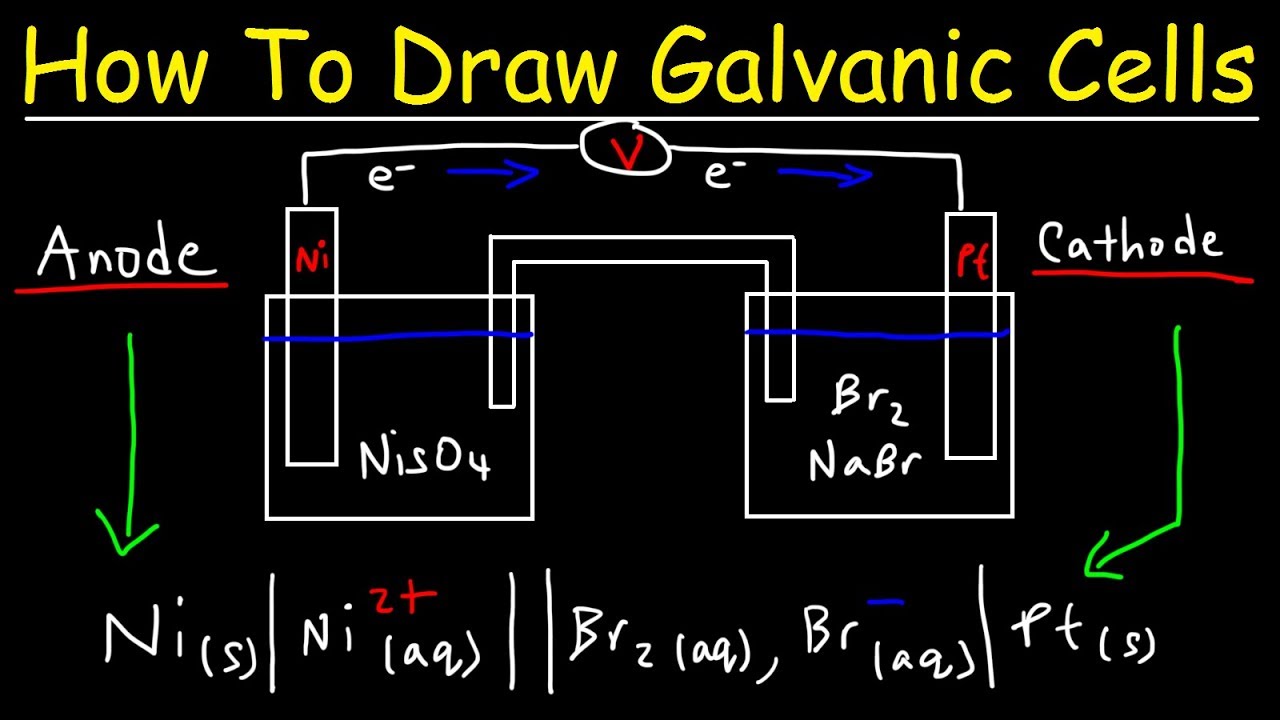
SOLVED: Suppose the galvanic cell sketched below is powered by the following reaction: Ni(s)-+CuSO 4(aq) NiSO4(aq)+Cu(s) S1 S2 Write balanced equation for the half- reaction that happens at the cathode of this

Sketch a voltaic cell for this redox reaction: Ni^{2+} (aq) + Mg (s) to Ni (s) + Mg^{2 +}(aq) a. Label the anode and cathode. b. Write the half reactions. c. Indicate

Example of a student generated Mg–Fe galvanic cell in the model kit,... | Download Scientific Diagram
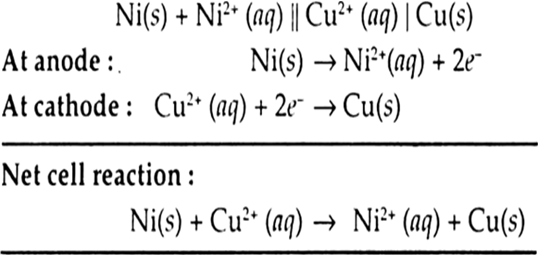
E0Ni2+/Ni and E0Cu2+/Cu are -0.25 V and + 0.34 respectively at 298 K. Formulate the self operating galvanic cell for this electrode pair. What reaction takes place in its operation? How is