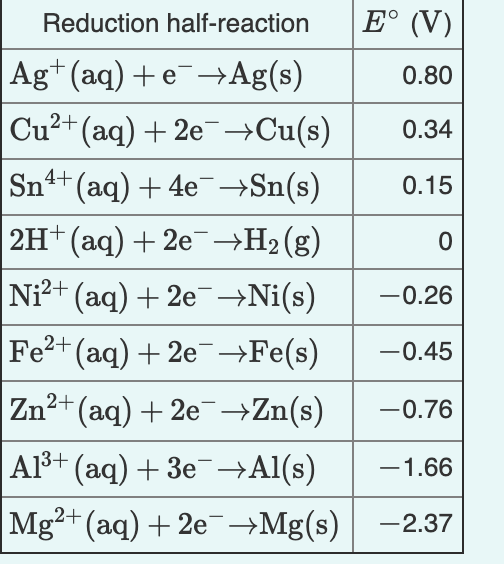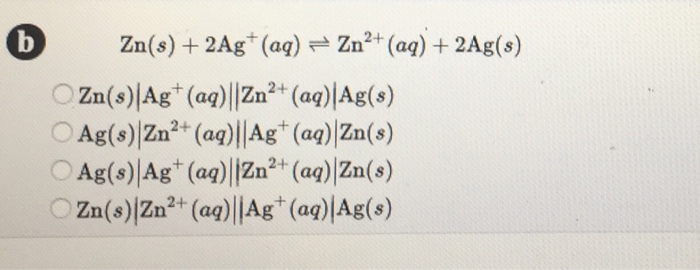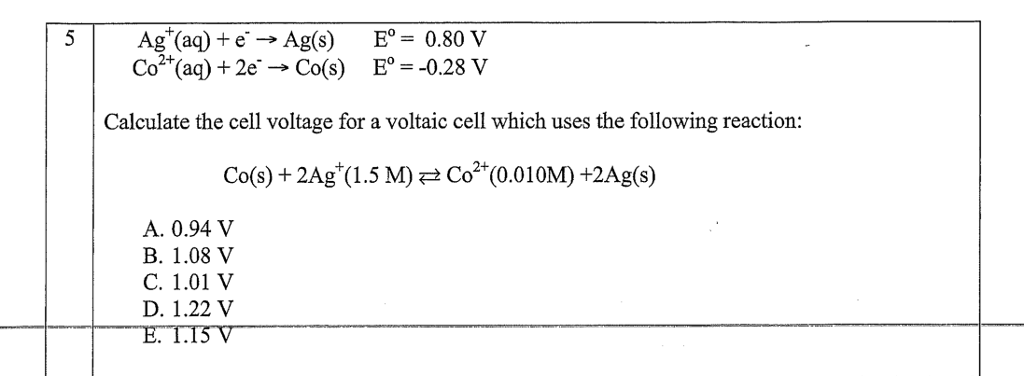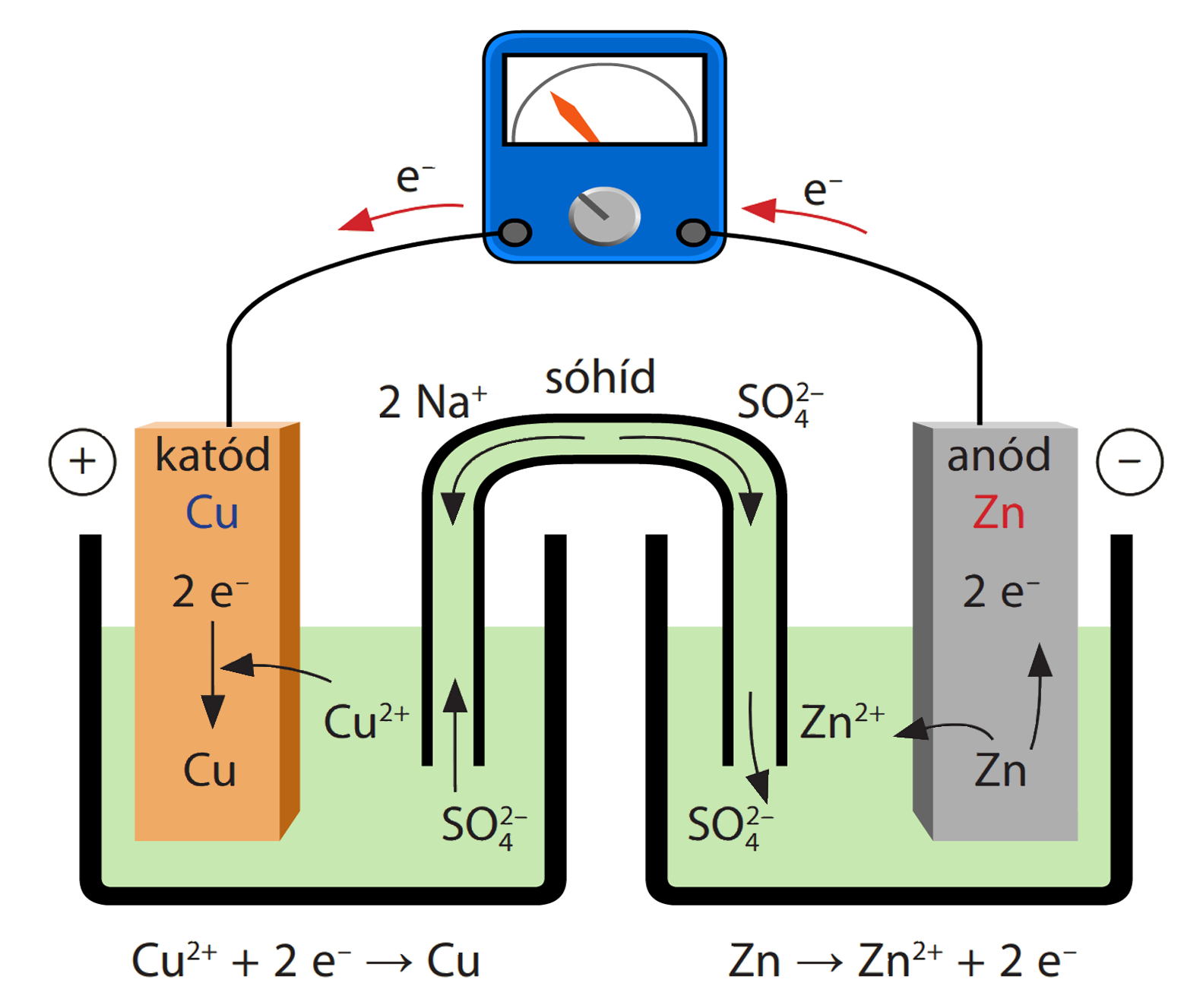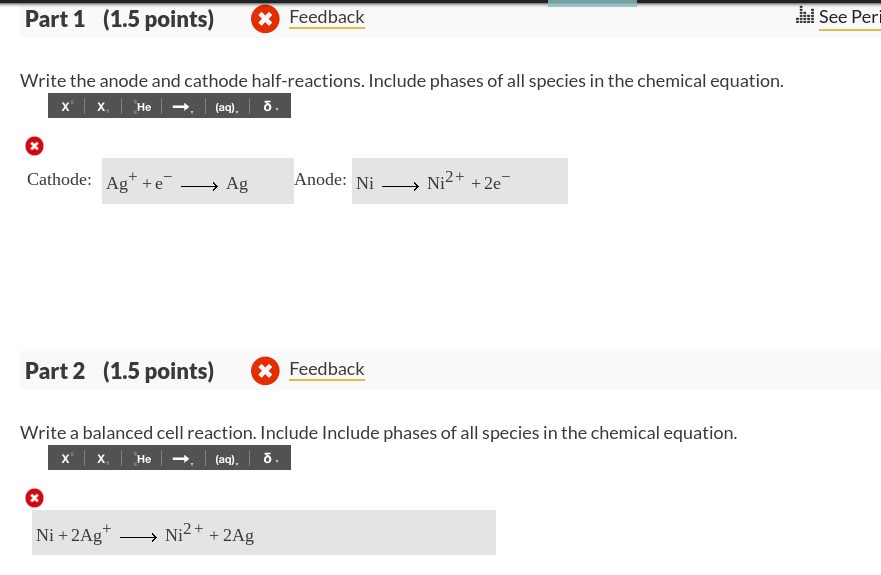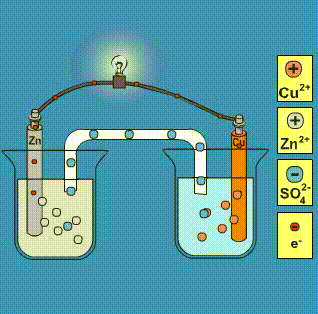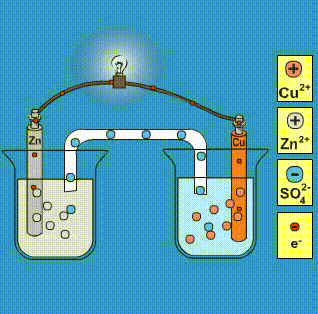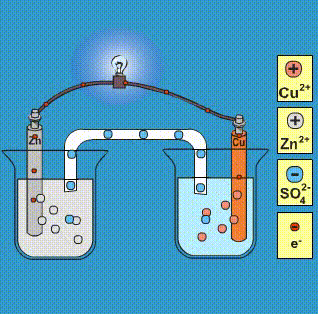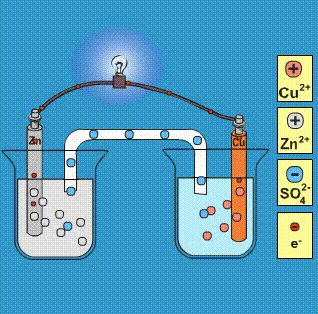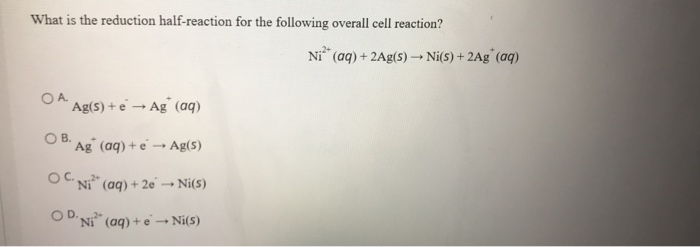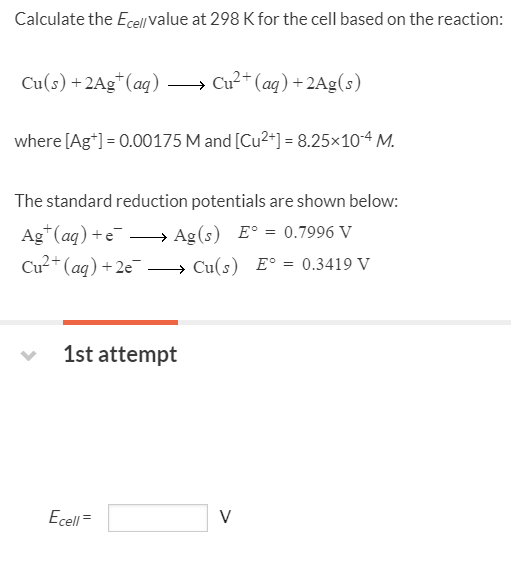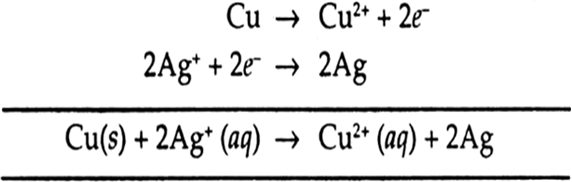
Cu2+ + 2e– → Cu E° = + 0.34 VAg+ + 1e– → Ag E° = + 0.80 V(i) Construct a galvanic cell using the above data.(ii) For what concentration of Ag+ ions

Given that,' ENi^2 + / Ni^∘ = - 0.25 V, ECu^2 + / Cu^∘ = + 0.34 V EAg^2 + / Ag^∘ = + 0.80 V, EZn^2 + / Zn^∘ = - 0.76 V Which of the following redox processes will not take place in specified direction?